Science and Innovation Boosts Infection Control
Partner with OEM to optimize your processes

It is the objective of all healthcare providers to deliver microbial free linen to the facilities who utilize these items in the care of their patients. The industry has found that there are many variables, and process steps that come into play when considering all that takes place between the time soiled linen is removed from the healthcare facility and returned clean, and ready for use once again. In each of these steps there are critical processing requirements and safeguards that must be adhered to so that bacteria is not transferred to clean environments, and that soiled linen is cleaned and kept microbial free until it returns back to the using facility.
Science plays a significant role in each of these steps, and this is certainly the case when looking at the processing of laundry. There are clearly defined operating parameters; which ideally remove soil and bacteria from linen. However, in doing so there are additional requirements for the laundry to make certain that the equipment, and processing environments do not become incubators for the growth, and manifestation of microbials. Regardless of processing environments the science still applies, and it is incumbent upon the laundry operation to ensure that they maintain a clean environment through strict adherence to preventative maintenance, cleaning and process management practices. Infection control in the laundry starts and ends with proper facility design, operational disciplines, adherence to the time tested scientific facts that surround the processing environment, and the validation of process and product compliance to established bacteria inspection protocols.
As an OEM we also play a vital role in the infection control process. Our focus is to apply the proper material, chemical and operational science to the design and functionality of our equipment to afford the laundry-processing environment the greatest level of flexibility to meet the challenges that this task presents. Specifically, Braun’s philosophy has always been, and will continue to be, to provide the most capable process regardless of equipment type and/or task.
Wash Pie
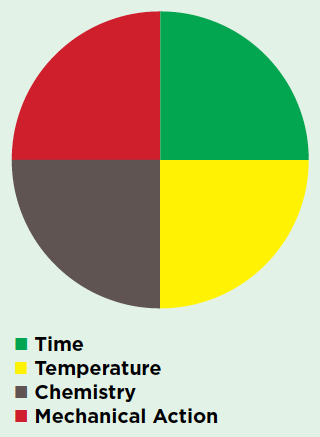
Scientific Facts
There are four main components required for proper linen processing. Changes in one of these requires that one or more of the others compensate for this change. This figure represents the optimum balance as there are benefits and liabilities associated with altering each of these variables. A failure to compensate for changes to the wash pie will result in one or more of the following:
- Poor wash quality/high re-wash % and a loss of production capacity
- Inadequate rinsing and neutralization
- Linen discoloration and decreased linen life
- Potentially excessive chemical consumption
In so doing, we believe that the solutions must be durable, easy to use and maintain, and that they must be based on proven science as opposed to marketing.
The greatest focus in the industry recently has been on the batch tunnel processing environment given the unique capabilities, and liabilities, that said systems can present when discussing infection control. It is clear that these systems provide exceptional and efficient productivity (regardless of OEM), however, they also can present a greater liability if material science, and proven wash pie relationships are compromised.
In the case of Braun we have provided the following differentiated features on all of our batch tunnel washers:
- Mono-Shell Open Helicoid tube design affords open pocket-like mechanical action and perpetual cleaning of the wash cylinder.
- The tube, all tanks and all process piping are made of stainless steel. Stainless steel is non-porous and by its nature minimizes the growth of biofilms.
- No dead spaces or surfaces that are not being mechanically cleaned with each batch transfer within the tunnel … which virtually eliminates biofilm accumulation.
- No stagnant bath or outer drum surfaces which can become incubation chambers for microbial growth.
- All seals utilized are peroxide cured to afford exceptional durability, resistance to temperature, chemical attack and biofilm production.
- All seals are accessible for cleaning, and preventative maintenance protocols.
- Heating systems afford the ability to support all processing chemistries and system cleaning demands.
- The data management solution included with the systems provides the ability to monitor critical process parameters.
Even with all of the captive design features noted the onus still falls on the end user to ensure that the appropriate facility design, and operational disciplines are in place throughout each step from the hospital and back again. Like with any process, it is critical that every input and process control for each step between these two locations gets an equal level of attention. If the inputs are bad, the operational disciplines are lacking, and there is a lack of validation regarding cleanliness there can, and most often will be, periods of non-conformance. It should be noted as well that processing facilities should not rely on the dryer, or ironer to eradicate bacteria, pathogens or microbials. Quality and system controls starts by controlling system inputs.
It is highly recommended that processing facilities consult with their OEMs, and leverage the knowledge that they possess to make certain that they are maximizing the capabilities of the solutions that they have been provided with! Although this month’s publication is focused on the healthcare market space, the content and subject matter applies with any of the laundry markets that our industry serves.